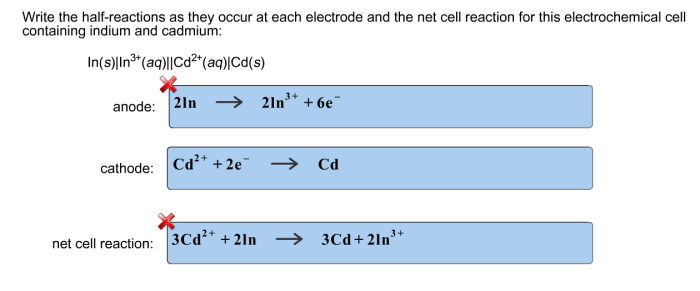
Write the half reactions as they occur at each electrode is a crucial concept in electrochemistry, providing the foundation for understanding the intricate workings of electrochemical cells. This concept delves into the fundamental principles of oxidation and reduction, unraveling the mechanisms that drive electrochemical reactions and shape the behavior of electrochemical systems.
Electrochemical cells, the heart of many energy-conversion technologies, rely on the interplay of half-reactions occurring at their respective electrodes. These half-reactions, representing the transfer of electrons between reactants and electrodes, hold the key to comprehending the overall electrochemical process and its applications in diverse fields such as batteries, fuel cells, and electroplating.
Electrochemical Cell

Electrochemical cells are devices that convert chemical energy into electrical energy or vice versa. They consist of two electrodes, an anode and a cathode, immersed in an electrolyte solution.The anode is the electrode where oxidation occurs, while the cathode is the electrode where reduction occurs.
Oxidation is the loss of electrons, while reduction is the gain of electrons.
Half-Reactions
Half-reactions are the individual oxidation and reduction reactions that occur at the anode and cathode, respectively. They are written as balanced equations that show the transfer of electrons between the reactants and products.Common half-reactions include:
Oxidation
Fe → Fe2+ + 2e-
Reduction
Cu2+ + 2e- → Cu
Oxidation and Reduction
Oxidation is the process of losing electrons, while reduction is the process of gaining electrons. In an electrochemical cell, oxidation occurs at the anode, while reduction occurs at the cathode.The anode is the negative electrode, while the cathode is the positive electrode.
Electrons flow from the anode to the cathode through the external circuit.
Balancing Half-Reactions
Half-reactions must be balanced in both mass and charge before they can be combined to form a complete electrochemical cell reaction.In acidic solutions, half-reactions can be balanced using the half-reaction method. This method involves adding electrons to the oxidation half-reaction and removing electrons from the reduction half-reaction until the total number of electrons lost and gained is equal.In
basic solutions, half-reactions can be balanced using the oxidation number method. This method involves assigning oxidation numbers to each element in the half-reaction and then adjusting the coefficients of the reactants and products until the total oxidation number change is equal to the number of electrons lost or gained.
Electrode Potentials
Electrode potential is a measure of the tendency of an electrode to undergo oxidation or reduction. It is measured in volts and is relative to a standard hydrogen electrode (SHE).The electrode potential of an electrode depends on several factors, including the nature of the electrode material, the concentration of the electrolyte solution, and the temperature.
Cell Potential
Cell potential is the difference in electrode potential between the anode and the cathode. It is measured in volts and is a measure of the driving force of the electrochemical cell reaction.The cell potential of an electrochemical cell is determined by the difference in the standard electrode potentials of the anode and cathode half-reactions.
Applications of Electrochemistry, Write the half reactions as they occur at each electrode
Electrochemistry has a wide range of practical applications, including:
Batteries
Batteries store electrical energy in chemical form and release it when needed.
Fuel cells
Fuel cells convert chemical energy from a fuel, such as hydrogen or natural gas, into electrical energy.
Electroplating
Electroplating is a process of coating a metal with another metal by electrolysis.
Expert Answers: Write The Half Reactions As They Occur At Each Electrode
What is the significance of half-reactions in electrochemistry?
Half-reactions provide a detailed understanding of the electron transfer processes occurring at each electrode, allowing us to analyze and predict the behavior of electrochemical cells.
How are half-reactions balanced in acidic and basic solutions?
Balancing half-reactions involves adjusting the number of electrons, protons, and water molecules to ensure the conservation of mass and charge in both acidic and basic solutions.
What factors influence electrode potentials?
Electrode potentials are affected by factors such as the nature of the electrode material, the concentration of reactants and products, and the temperature of the solution.